Abstract
Although survival rate for children with Acute Lymphoblastic Leukemia (ALL) now exceeds about 90%, the outcome of adult patients with ALL is extremely poor. These differences might be attributed to the lack of insights into pathogenesis and clinical behavior of adult-ALL.
Gross chromosomal alterations including chromosome translocations and aneuploidy are considered as early events in ALL and constitute disease subtypes. To identify chromosome translocations underlying adult with Ph-negative B-ALL, we performed RNA-seq analysis on RNA from individuals with B-ALL who had been treated on the Japan Adult Leukemia Study Group (JALSG) ALL202-O protocol (n = 149).
We successfully identified chromosome translocations in 100 patients (67.1%). ZNF384 fusions were most frequently detected in 30 patients (20.1%) and they had wide range of fusion partners. DUX4- and MEF2D- fusions were also recurrently found in 7 (4.7%) and 9 (6.0%) patients, respectively. Chromosome translocations activating kinase and cytokine receptor were found in 25 patients (16.8%) with Ph-like gene expression profile. These alterations were almost completely mutually exclusive indicating these are likely to be primary genetic events.
For simplicity, here we define (1) fusions involving ZNF384, DUX4, MEF2D, CEBP and PAX5 as well as TCF3-PBX1 and ETV6-RUNX1 as Transcription Factor fusions (TF fusions; 49% of patients), (2) fusions involving CRLF2, JAK2, PDGFRB, EPOR and ABL as Kinase/cytokine-receptor Activating fusions (KA fusions; 15%) and (3) non-recurrent fusions or the absence of fusions/aneuploidy as B-others (30%).
First, we analyzed impact of the patient age on types of fusion genes, based of combined data of ALL202-O cohort, childhood B-ALL cohort (Lilljebjörn H, et al. 2016: n = 189) and ALL202-U cohort (Yasuda T, et al. 2016: n = 54). We found that incidence of ZNF384-, CEBP- fusions and B-others increases as patients age, whereas ETV6-RUNX1 and PAX5 fusions were more prevalent in younger patients, exhibiting negative association with age. DUX4 fusions and TF fusions were most prevalent in Adolescent and Young Adult (AYA) generation. JAK2-, PDGFRB-, EPOR- and KA- fusions were positively correlated with age.
Next, we analyzed association between patient survival and types of fusions. In Japanese adult B-ALL cohort (ALL202-O and ALL202-U cohort), we observed ZNF384-, DUX4- fusions and TCF3-PBX1 were associated with better disease-free survival than B-others. Furthermore, when combined, MEF2D- (n = 14), CEBP- (n = 4), PAX5- fusions (n = 2) and ETV6-RUNX1 (n = 2) exhibited significantly better disease-free survival than B-others, indicating TF fusions were associated with an improved outcome. In contrast, KA fusions were associated with poorer disease-free survival than B-others. KMT2A fusions were comparable with B-others regarding to patient disease-free survival.
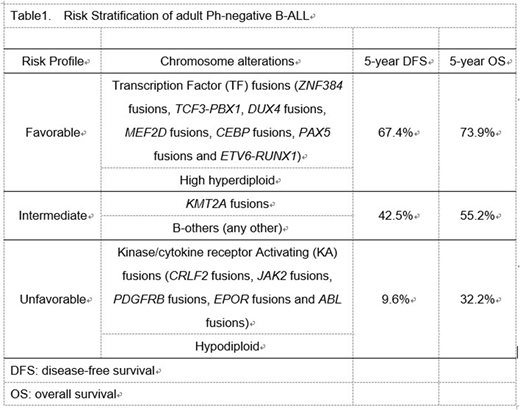
These results allowed us to develop a prognostic schema to identify three distinct risk profile groups, based on types of fusion genes and cytogenetics (Table1); favorable-risk (5-year rate of disease-free survival 67.4%), intermediate-risk (5-year rate of disease-free survival 42.5%) and adverse-risk (5-year rate of disease-free survival 9.6%). This prognostic schema predicted the outcome independently of age, sex and methotrexate dose in multivariate analysis (p < 0.001).
In conclusion, we promoted a better understanding of the genetic basis of adult B-ALL by focusing on fusion genes. Each chromosome translocations were closely associated with age. ZNF384-, KA fusions and B-others were characteristic for older-adult patients (40-65 years old) with B-ALL. We clearly demonstrated specific primary chromosome abnormalities are strong prognostic marker. Functional properties of primary genetic events (TF fusions vs. KA fusions) might be a key determinant of biological characteristics and clinical outcome.
Kiyoi:Novartis Pharma K.K.: Research Funding; Celgene Corporation: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; FUJIFILM Corporation: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Bristol-Myers Squibb: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; Sanofi K.K.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Phizer Japan Inc.: Research Funding; Eisai Co., Ltd.: Research Funding. Naoe:Astellas Pharma Inc.: Research Funding; Fujifilm Corporation: Patents & Royalties, Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Pfizer Japan Inc.: Research Funding; Toyama Chemical Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal